The idea that once a painting dries it will be stable and stop reacting with its environment is an appealing one. One can easily imagine the work hanging innocently on a gallery or museum wall, its surface luminous and smooth, or thick and pastose, gleaming into the indefinite future. However, the reality of a painting’s lifetime is unfortunately quite different, and much messier. Paintings are continuously reacting with their environments (a phenomenon readily observable by, for example, the craquelure of works on panel). This includes being damaged over the long term by even the most unavoidable ‘agents of degradation’ such as ordinary visible light, humidity, and oxygen. Our new age of understanding the fragility of oil paintings began in 1999 when Petria Noble, then a paintings conservator at the Mauritshuis in The Hague (and now at the Rijksmuseum), noticed the presence of small nodules (similar to grains of sand) forming in Rembrandt’s The Anatomy Lesson of Dr Nicolaes Tulp (1632). These little bumps in the paint layers were revealed by scientific study to be the painting’s oil binder reacting with lead-based pigments such as lead white or lead-tin yellow.
The pigments and paint binder react with each other over centuries to form a type of large molecule known as a ‘lead soap’. In and of themselves, lead soaps can cause paint films to appear darker and more transparent as a painting ages, especially when they are well-distributed throughout the painting. This increased transparency is a well-known phenomenon in Old Master paintings. Over time, the lead soaps can gather together into little spheres that grow inside of a paint layer until they ultimately erupt through the surface of the painting. This causes the painting to break out with little raised white spots, sometimes over large areas of its surface (in a phenomenon that has recently been called ‘art acne’ or, as I prefer, ‘masterpiece measles’). When it was first identified this unfortunate disease was thought to be a problem only for Old Masters, a result of the advanced age of these sublime works. Then along came Silvia Centeno, a chemist at the Metropolitan Museum of Art, who discovered lead soap measles popping out in the museum’s beloved Portrait of Madame X by John Singer Sargent, from 1883–84. The observation of lead soap protrusions in this work made scientists and art conservators nervous that the phenomenon was more widespread than we had previously thought.
Click on the image to view an animated GIF of a magnified section of Georgia O’Keeffe’s Pedernal that isolates micron-sized protrusions from metal soaps to identify the presence of ’art acne’. Photo: Dale Kronkright/© Georgia O’Keeffe Museum
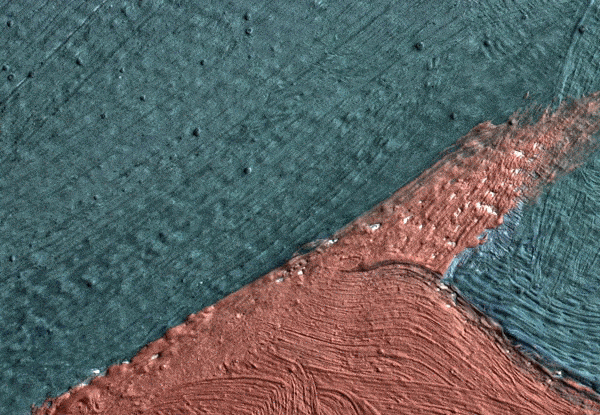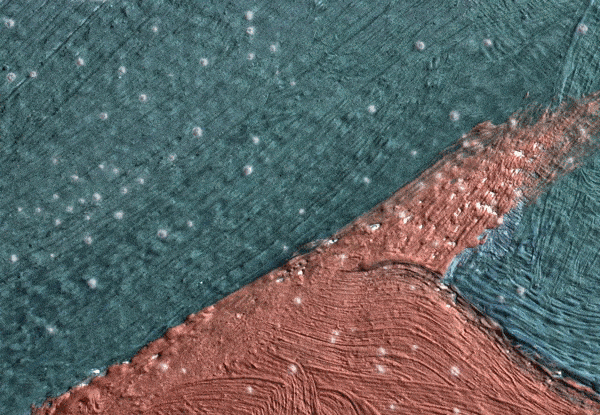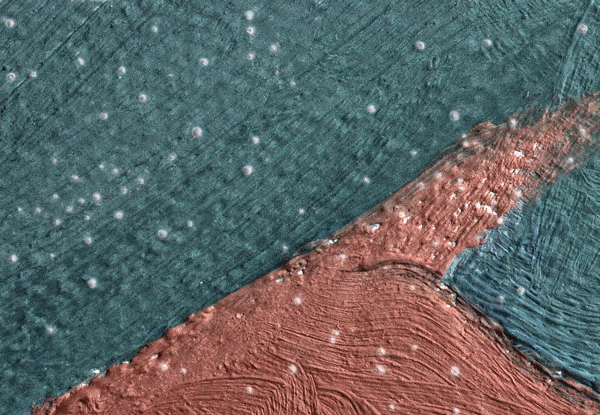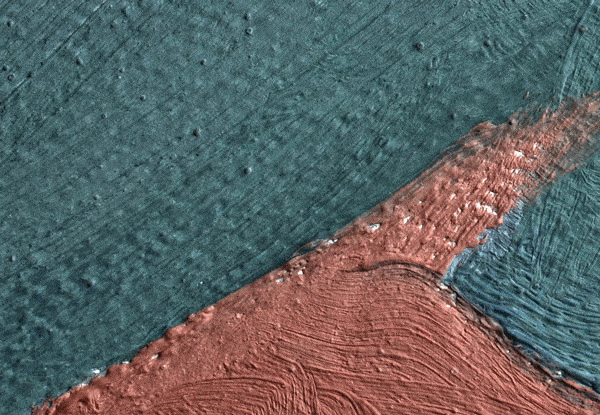
Now we know that the phenomenon is not restricted to lead paints. With the introduction of zinc white paints in the early 19th century, the works of the Impressionists through to the Abstract Expressionists are being plagued by the formation of zinc soaps. Zinc soap formation can result in the delamination of the painting from its zinc white ground layer, where the uppermost paint layers literally lift up and flake off of the canvas. These alarming occurrences have been observed in works by the 20th-century artists Franz Kline, Piet Mondrian and Salvador Dalí. This week, Marc Walton and his colleagues from Northwestern University reported on the phenomenon in works by Georgia O’Keeffe. Walton’s team has also revealed that as many as seven out of 10 paintings are inherently unstable as a result of metal soaps. These works are, in unfavourable environmental conditions, good candidates for the measles or delamination.
Now that the gravity of the situation is understood, what do we do next? House our prized painting collections under argon gas? Visit our favourite museums in scuba suits? Certainly these findings demonstrate the critical need for funding for scientific and art conservation research. Fortunately, Walton’s work involves getting to a deeper understanding of how a painting interacts with its environment to accelerate or delay the growth of metal soaps. Whether you call it art acne, masterpiece measles or (as we call it in the business) molecular self-assembly, stay tuned for updates on how to avoid these unflattering molecular rearrangements in your favourite Van Gogh.
Jennifer Mass is the Andrew W. Mellon Professor of Cultural Heritage Science at the Bard Graduate Center, New York.